Gendarussa
Gendarussa (sometimes spelled gandarusa) is an herbal male contraceptive. It’s derived from a three-meter-high shrub that grows natively in Indonesia on the island of Papua. The company PT Indo Farma has successfully extracted the active ingredient from the plant and produced a contraceptive in pill form.
The researchers who developed the pill suspect that gendarussa affects the enzymes which allow sperm to penetrate an ovum. Without these enzymes working properly, the sperm will not be able to get through the ovum’s outer shell. Preliminary findings have not shown any side effects.
 Professor Prajogo, the leading researcher on gendarussa, has studied it since 1985. He’s led the drug through the first two phases of clinical trials.In 2009, the phase-one study had 32 single men take the extract for 144 days. The 2010 phase-two study had 80 of 120 married men take the extract for 102 days. In 2012, the research team added an additional phase-two study which had 186 of 350 married men take the pill for 30 days. Only one pregnancy occurred during the trials.
Professor Prajogo, the leading researcher on gendarussa, has studied it since 1985. He’s led the drug through the first two phases of clinical trials.In 2009, the phase-one study had 32 single men take the extract for 144 days. The 2010 phase-two study had 80 of 120 married men take the extract for 102 days. In 2012, the research team added an additional phase-two study which had 186 of 350 married men take the pill for 30 days. Only one pregnancy occurred during the trials.
Recent efforts have focused on having gendarussa registered as a new drug by the Indonesian FDA. Phase-3 research is now needed to confirm the effectiveness shown in the phase-2 studies and to evaluate safety in a larger number of men over a longer time period. The results of phase-3 research will also determine gendarussa’s duration of action and dosage.
Getting gendarussa approved in the US would likely be a significant hurdle. Much of the research would need to be repeated to comply with U.S. regulations.
Research Articles
- Handayani, L. (2011). A Male Contraceptive Pill Consist of Gandarusa (Justicia gendarussa Burm. F). Journal of the Indonesian Medical Association, 57(08). [Text] [Translated]
- Kiren, Y., Deguchi, J., Hirasawa, Y., Morita, H., & Prajogo, B. (2014). Justidrusamides A–D, new 2-aminobenzyl alcohol derivatives from Justicia gendarussa. Journal of Natural Medicines, 1-5.
- Leaves, J. G. B. F. (2009). ISOLATION OF MALE ANTIFERTILITY COMPOUND IN N-BUTANOL FRACTION OF. Folia Medica Indonesiana, 45(1), 28-31. [Text]
- Prajogo, B., Nasronudin, Cholies, N., and Neny, P. (2010). Development of Justicia Gendarussa Burm.f as Anti-HIV. Joint Symposium emerging & Re-emerging Infections Diseases Update III. June 19-20. [Abstract Text]
- Prajogo, B. (2014). American Society of Andrology and European Academy of Andrology. [Additional Abastracts]
- Sihabuddin, M., Maria, A., JS, F., Pramesti, B., Musta’ina, S., Radjaram, A., … & Prajogo EW, B. (2013). PHARMACOKINETIC PARAMETERS DETERMINATION OF GENDARUSIN A IN MEN SUBJECT URINE AFTER ADMINISTRATION OF ETHANOL EXTRACT OF Justicia gendarussa Burm. f. LEAF (ETHNO MEDICINE RESEARCH). Jurnal Medika Planta, 1(4). [Text]
Eppin
Eppin is actually the target for an anti-Eppin drug that could be delivered either orally or potentially beneath the skin. Delivery through a medical device beneath the skin would have the benefit of removing user error, thus decreasing pregnancy rates significantly. But this might not be feasible.
During normal ejaculation, semenogelin, a protein found in seminal fluid, binds to Eppin, a protein covering the sperm’s surface. This binding inhibits sperm movement until a particular enzyme, PSA, removes the semenogelin protein. After the enzyme removes this protein, the sperm can swim towards the egg.
The anti-Eppin drug works by binding to Eppin on the sperm’s surface. The anti-Eppin drug binding to Eppin mimics the effect of semenogelin binding to Eppin—it stops the sperm from swimming. And, importantly, the PSA enzyme doesn’t remove it. Because the anti-Eppin drug remains bound to Eppin, the sperm aren’t able to swim towards the egg.

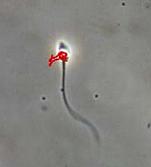 This approach to limit sperm motility has been studied since the mid 2000’s. The anti-Eppin drug has been found to work on human sperm and researchers have testable lead compounds ready to go. The next steps involve the toxicology testing which the FDA requires in order for the drug to move into Phase 1 clinical trials.Dr. Michael O’Rand is the lead developer of Eppin.
This approach to limit sperm motility has been studied since the mid 2000’s. The anti-Eppin drug has been found to work on human sperm and researchers have testable lead compounds ready to go. The next steps involve the toxicology testing which the FDA requires in order for the drug to move into Phase 1 clinical trials.Dr. Michael O’Rand is the lead developer of Eppin.
Research Articles
- Clauss A, Lilja H, Lundwall A. A locus on human chromosome 20 contains several genes expressing protease inhibitor domains with homology to whey acidic protein. Biochem J. 2002;368:233–242.
- Denolet E, De Gendt K, Allemeersch J, Engelen K, Marchal K, Van Hummelen P, Tan KAL, Sharpe RM, Saunders PTK, Swinnen JV, Verhoeven G. The effect of a Sertoli cell–selective knockout of the androgen receptor on testicular gene expression in prepubertal mice. Mol Endocrinol. 2006;20:321–334.
- Ding X, Zhang J, Juan Fei J, Bian Z, Li Y, Xia Y, Lu C, Song L, Wang S, Wang X. Variants of the EPPIN gene affect the risk of idiopathic male infertility in the Han-Chinese population. Hum Reprod. 2010;25:1657–1665.
- Hamil KG, Sivashanmugam P, Richardson RT, Grossman G, Ruben SM, Mohler JL, Petrusz P, O’Rand MG, French FS, Hall SH. HE2b and HE2g, new members of an epididymis-specific family of androgen-regulated proteins in the human. Endocrinology. 2000;141:1245–1253.
- Metz CB. Immunological inhibition of sperm and egg function: prospects for an antifertility vaccine from gametes. In: Contraception: Science, Technology, and Application. Proceedings of a Symposium. Washington, DC: National Academy of Sciences; 1979:180–220.
- Mitra A, Richardson RT, O’Rand MG. Analysis of recombinant human semenogelin as an inhibitor of human sperm motility. Biol Reprod. 2010;82:489–498.
- O’Rand MG, Widgren EE, Beyler S, Richardson RT. Inhibition of human sperm motility by contraceptive anti-eppin antibodies from infertile male monkeys: effect on cAMP. Biol Reprod. 2009; 80:279–285.
- O’Rand MG, Widgren EE, Sivashanmugam P, Richardson RT, Hall SH, French FS, VandeVoort CA, Ramachandra SG, Ramesh V, Rao AJ. Reversible immunocontraception in male monkeys immunized with eppin. Science. 2004;306:1189–1190.
- O’Rand MG, Widgren EE, Wang Z, Richardson RT. Eppin: an effective target for male contraception. Mol Cell Endocrinol. 2006;250:157–162.
- Page ST, Amory JK, Bremner WJ. Advances in male contraception. Endocrine Rev. 2008;29:465–493. Richardson RT, Sivashanmugam P, Hall SH, Hamil KG, Moore PA, Ruben SM, French FS, O’Rand MG. Cloning and sequencing of human Eppin: a novel family of protease inhibitors expressed in the epididymis and testis. Gene. 2001; 270:93–102.
- Robert M, Gibbs BF, Jacobson E, Gagnon C. Characterization of prostate-specific antigen proteolytic activity on its major physio-logical substrate, the sperm motility inhibitor precursor/semenogelin I. Biochemistry. 1997;36:3811–3819.
- Schauwaers K, De Gendt K, Saunders PTK, Atanassova N, Haelens A, Callewaert L, Moehren U, Swinnen JV, Verhoeven G, Verrijdt G, Claessens F. Loss of androgen receptor binding to selective androgen response elements causes a reproductive phenotype in a knockin mouse model. Proc Natl Acad Sci U S A. 2007; 104:4961–4966.
- Wang Z, Widgren EE, Richardson RT, O’Rand MG. Characterization of an eppin protein complex from human semen and spermatozoa. Biol Reprod. 2007a;77:476–484. Wang Z, Widgren EE, Richardson RT, O’Rand MG. Eppin: a molecular strategy for male contraception. Soc Reprod Fertil Suppl. 2007b;65:535–542.
- Wang Z, Widgren EE, Sivashanmugam P, O’Rand MG, Richardson RT. Association of eppin with semenogelin on human spermatozoa. Biol Reprod. 2005;72(4):1064–1070.
- Willems A, De Gendt K, Allemeersch J, Smith LB, Welsh M, Swinnen JV, Verhoeven G. Early effects of Sertoli cell–selective androgen receptor ablation on testicular gene expression. Inter J Androl. 2009;33:507–517.
- Yenugu S, Richardson RT, Sivashanmugam P, Wang Z, O’Rand MG, French FS, Hall SH. Antimicrobial activity of human EPPIN, an androgen regulated sperm bound protein with a whey acidic protein motif. Biol Reprod. 2004;71:1484–1490.
Clean Sheets Pill
The “clean sheets pill” acts by relaxing the longitudinal mucles in the vas deferens while still allowing the circular muscles to contract. This relaxation prevents the semen-carrying tubes from pushing the sperm forward.
This method is extremely fast acting, needing to be taken only a few hours before intercourse. Its effects would then last 16-24 hours. This method may also be implemented by a time-release implant. An implant would sidestep user error and effectively lower the real-world pregnancy rate.
The drug the pill is based on, phenoxybenzamine, has some side effects. But the prototype contraceptive pills have been designed to avoid these effects and isolate its muscle-relaxing function. The drug’s function also seems to affect accessory glands so that they do not contribute seminal fluid—hence the name “clean sheets pill.” If the drug prevents all fluids from being ejaculated, it will have the secondary benefit of reducing the risk of spreading semen-borne STI’s.
Orgasmic function continues as usual.
 The “clean sheets pill” was developed by Dr. Nnaemeka Amobi with Dr. Christopher
The “clean sheets pill” was developed by Dr. Nnaemeka Amobi with Dr. Christopher  Smith at King’s College of London. The research team is collaborating with Professor John Guillebaud, Emeritus Professor of Family Planning & Reproductive Health at University College London. Together, they’re seeking funding to begin the next phase of the study using rams. The drug is still being optimized to achieve 100% semen inhibition.
Smith at King’s College of London. The research team is collaborating with Professor John Guillebaud, Emeritus Professor of Family Planning & Reproductive Health at University College London. Together, they’re seeking funding to begin the next phase of the study using rams. The drug is still being optimized to achieve 100% semen inhibition.
Research Articles
- Amobi, NI, J Guillebaud, AV Kaisary, E Turner and IC Smith (2002) “Discrimination by SZL49 between contractions evoked by noradrenaline in longitudinal and circular muscle of human vas deferens.” British Journal of Pharmacology 136(1):127-35.
- Amobi, N, J Guillebaud, A Kaisary, RW Lloyd-Davies, E Turner and IC Smith (2003) “Contractile actions of imidazoline alpha-adrenoceptor agonists and effects of noncompetitive alpha1-adrenoceptor antagonists in human vas deferens.” European Journal of Pharmacology 462(1-3):169-77. Erratum in: 464(2-3):241.
- Amobi, NI, IP Chung and IC Smith (2006) “Attenuation of contractility in rat epididymal vas deferens by Rho kinase inhibitors.” Autononmic & Autacoid Pharmacology 26(2):169-81.
- Hisasue, S, R Furuya, N Itoh, K Kobayashi, S Furuya and T Tsukamoto (2006) “Ejaculatory disorder caused by alpha-1 adrenoceptor antagonists is not retrograde ejaculation but a loss of seminal emission.” International Journal of Urology 13(10):1311-6.
- Kedia, KR, and L Persky (1981) “Effect of phenoxybenzamine (dibenzyline) on sexual function in man.” Urology 18(6):620-1.
Vasalgel
Vasalgel is a hydrogel polymer that’s injected into each of the man’s vas deferens, the tubes that carry sperm from the epididymis to the ejaculatory ducts. The polymer works by blocking sperm, although fluid can still pass so that there is no build-up of pressure. Vasalgel does not affect hormones or ejaculation. Vasalgel’s insertion is done through a practically painless 15-minute no-scalpel procedure using local anesthesia. Vasalgel is reversed through a second injection which dissolves and flushes out the polymer.

Vasalgel is being developed by Revolution Contraceptives, LLC – a social venture under the umbrella of nonprofit Parsemus Foundation. The organization aims to release Vasalgel to a broad market with affordable yet sustainable pricing once regulatory requirements are met.
Vasalgel is currently undergoing animal trials in the U.S. The project has produced a successful 12-month rabbit study which resulted in a rapid onset of infertility after injection with Vasalgel. The rabbits’ sperm then returned once the polymer was flushed out. Additional research on reversal methodology is ongoing while preparations for the first clinical trial are underway. Vasalgel is expected to be tested as a medical device.
Vasalgel is inspired from work on Reversible Inhibition of Sperm Under Guidance (RISUG), which also uses a polymer injected into the vas deferens. This approach was developed and patented in India by Dr. Sujoy Guha. The progress on RISUG is unclear at this time but has gone into Phase III human clinical trials.
Research Articles
- Ali BH. “Dimethyl sulfoxide: recent pharmacological and toxicological research.” Vet Hum Toxicol. 2001 Aug; 43(4): 228-31. Review. PMID: 11474739 [PubMed – indexed for MEDLIN
- Assessment of Polystyrene (SMA) in Bead Form as a Bio-Implant. Unpublished; available from Prof. Sujoy K. Guha.
Chaki SP, Das HC, Misro MM. “A short-term evaluation of semen and accessory sex gland function in phase III trial subjects receiving intravasal contraceptive RISUG.” Contraception. 2003 Jan; 67(1): 73-8. PMID: 12521662 [PubMed – indexed for MEDLIN - Chaube SK, Misro MM. “Effect of styrene maleic anhydride (SMA) on viability and integrity of isolated rat oocytes in vitro.” Contraception. 2002 Dec; 66(6): 469-72. PMID: 12499041 [PubMed – in process]
Chaudhury K, Bhattacharyya AK, Guha SK. “Studies on the membrane integrity of human sperm treated with a new injectable male contraceptive.” Hum Reprod. 2004 Aug; 19(8): 1826-30. Epub 2004 Jun 10. PMID: 15192063 [PubMed – in process]
Chaudhury K, Sharma U, Jagannathan NR, Guha SK. “Effect of a new injectable male contraceptive on the seminal plasma amino acids studied by proton NMR spectroscopy.” Contraception. 2002 Sep; 66(3): 199-204. PMID: 12384210 [PubMed – indexed for MEDLIN - Guha SK, Anand S, Ansari S, Farooq A, Sharma DN. “Time-controlled injectable occlusion of the vas deferens.” Contraception. 1990 Mar; 41(3): 323-31. PMID: 2323220 [PubMed – indexed for MEDLIN
- Guha SK, Anand S. “Gamete transport mechanism.” In: Talwar GP, editor. Recent advances in reproduction and regulation of fertility. Amsterdam: Elsevier; 1979. p 211-16.
Guha SK, Ansari S, Anand S, Farooq A, Misro MM, Sharma DN. “Contraception in male monkeys by intra-vas deferens injection of a pH lowering polymer.” Contraception. 1985 Jul; 32(1): 109-18. PMID: 4053602 [PubMed – indexed for MEDLIN - Guha SK, Kaur H, Ahmed AM. “Mechanics of spermatic fluid transport in the vas deferens.” Med Biol Eng. 1975 Jul; 13(4): 518-22. No abstract available. PMID: 811934 [PubMed – indexed for MEDLIN
- Guha SK, Singh G, Anand S, Ansari S, Kumar S, Koul V. “Phase I clinical trial of an injectable contraceptive for the male.” Contraception. 1993 Oct; 48(4): 367-75. PMID: 8222664 [PubMed – indexed for MEDLIN
- Guha SK, Singh G, Ansari S, Kumar S, Srivastava A, Koul V, Das HC, Malhotra RL, Das SK. “Phase II clinical trial of a vas deferens injectable contraceptive for the male.” Contraception. 1997 Oct; 56(4): 245-50. PMID: 9408706 [PubMed – indexed for MEDLIN
- Guha SK, Singh G, Srivastava A, Das HC, Bhardwaj JC, Mathur V, Koul V, Malhotra RL and Das SK “Two-year clinical efficacy trial with dose variations of a vas deferens injectable contraceptive for the male.” Contraception 1998; 58:165-174.
Guha SK. “Contraceptive for use by a male.” United States Patent 5488075; Jan. 30, 1996.
Guha SK. “Current status of development and clinical testing of male intravasal contraceptive.” In: Rajalakshmi M, Griffin PD, editors. Male contraception: present and future. New Delhi: New Age International; 1998/1999. p 274-85.
Guha SK. “Electrical effects on mammalian sperm.” In: Engineering in Medicine and Biology Society, 1988. Proceedings of the Annual International Conference of the IEEE. Pub. Date: 4-7 Nov 1988; vol.4 p 1881-1882.
Guha SK. “Injectable contraceptive for the male.” Expert Opin Investig Drugs. 1995 June; 4(6): 537 43.
Guha SK. “Male fertility regulation by intra-vas deferens injection.” In: Moudgal NR, Yoshinaga K, Rao AJ, Adiga PR, editors. Perspectives in primate reproductive biology. Bangalore: Wiley Eastern Limited; 1991. p 345-51.
Guha SK. “Non-invasive reversal of intraluminal vas deferens polymer injection-induced azoospermia–technology.” Asian J Androl. 1999 Sep; 1(3): 131-4. PMID: 11250780 [PubMed – indexed for MEDLIN - Guha SK. “RISUG™ (Reversible Inhibition of Sperm Under Guidance) – An antimicrobial as male vas deferens implant for HIV free semen.” Med Hypotheses. 2005;65(1):61-4. PMID: 15893119 [PubMed – indexed for MEDLIN
- Guha, SK, Kaur H, Ahmed, AM. “Formulation of a fertilization index for male fertility studies.” Med Life Sci Eng. 1975; 1: 17-27.
Koshy LM. “Charging the vas deferens — a novel male contraceptive method.” (Review article) Indian Med Trib. 1994 Aug 30; 2(14): 6. PMID: 12179180 [PubMed – indexed for MEDLIN - Koul V, Ansari S, Sharma K, Anand S, Guha SK. “Dose-dependent relationship of polymeric hydrogels on motility and vitality of human spermatozoa in vitro.” Journal of Materials Science: Materials in Medicine. 1994 Apr; 6(4): 192-6.
Koul V, Srivastava A, Guha SK. “Reversibility with sodium bicarbonate of styrene maleic anhydride, an intravasal injectable contraceptive, in male rats.” Contraception. 1998 Oct; 58(4): 227-31. PMID: 9866004 [PubMed – indexed for MEDLIN - Kumar S, Chaudhury K, Sen P, Guha SK. “Atomic force microscopy: a powerful tool for high-resolution imaging of spermatozoa.” J Nanobiotechnology. 2005 Sep 27;3:9. PMID: 16188038 [PubMed – indexed for MEDLIN
- Lohiya NK, Manivannan B, Mishra PK, Pathak N, Balasubramanian SP. “Intravasal contraception with styrene maleic anhydride and its noninvasive reversal in langur monkeys (Presbytis entellus entellus).” Contraception. 1998 Aug; 58(2): 119-28. PMID: 9773267 [PubMed – indexed for MEDLIN
- Lohiya NK, Manivannan B, Mishra PK, Pathak N. “Non-invasive reversible vas occlusion with styrene maleic anhydride: a possible alternative to vasectomy.” In: Puri CP, Van Look PFA, editors.Sexual and reproductive health: recent advances, future directions. New Delhi: New Age Int (P) Ltd; 2001. p 249-68.
Lohiya NK, Manivannan B, Mishra PK, Pathak N. “Vas deferens, a site of male contraception: an overview.” Asian J Androl. 2001 Jun; 3(2): 87-95. PMID: 11404791 [PubMed – in process]
Lohiya NK, Manivannan B, Mishra PK, Sriram S, Bhande SS, Panneerdoss S. “Preclinical evaluation for noninvasive reversal following long-term vas occlusion with styrene maleic anhydride in langur monkeys.” Contraception. 2005 Mar;71(3):214-26. PMID: 15722073 [PubMed – indexed for MEDLIN - Lohiya NK, Manivannan B, Mishra PK. “Repeated vas occlusion and non-invasive reversal with styrene maleic anhydride for male contraception in langur monkeys.” Int J Androl. 2000 Feb; 23(1): 36-42. PMID: 10632760 [PubMed – indexed for MEDLIN
- Lohiya NK, Manivannan B, Mishra PK. “Ultrastructural changes in the spermatozoa of langur monkeys Presbytis entellus entellus after vas occlusion with styrene maleic anhydride.” Contraception. 1998 Feb; 57(2): 125-32. PMID: 9589840 [PubMed – indexed for MEDLIN
- Mahajan S, Guha SK, Misro MM. “A theoretical and scanning electron microscope study on the electrically mediated inactivation of spermatozoa.” J Biol Phys. (Journal of Biological Physics) 1982; 10: 112/113-123/124.
Manivannan B, Bhande SS, Panneerdoss S, Sriram S, Lohiya NK. “Safety evaluation of long-term vas occlusion with styrene maleic anhydride and its non-invasive reversal on accessory reproductive organs in langurs.” Asian J Androl. 2005 Jun;7(2):195-204. PMID: 15897977 [PubMed – indexed for MEDLIN - Manivannan B, Mishra PK, Lohiya NK. “Ultrastructural changes in the vas deferens of langur monkeys Presbytis entellus entellus after vas occlusion with styrene maleic anhydride and after its reversal.” Contraception. 1999 Feb; 59(2): 137-44. PMID: 10361629 [PubMed – indexed for MEDLIN
- Mishra PK, Manivannan B, Pathak N, Sriram S, Bhande SS, Panneerdoss S, Lohiya NK. “Status of Spermatogenesis and Sperm Parameters in Langur Monkeys Following Long-term Vas Occlusion With Styrene Maleic Anhydride.” J Androl. 2003 Jul-Aug; 24(4): 501-9. PMID: 12826690 [PubMed – indexed for MEDLIN
- Misro M, Guha SK, Singh H, Mahajan S, Ray AR, Vasudevan P. “Injectable non-occlusive chemical contraception in the male – I.” Contraception. 1979 Nov; 20(5): 467-73. PMID: 43211 [PubMed – indexed for MEDLIN
- Misro MM, Kaur H, Mahajan S, Guha SK. “An intravasal non-occlusive contraceptive device in rats.” J Reprod Fertil. 1982 May; 65(1): 9-13. PMID: 7077606 [PubMed – indexed for MEDLIN
- Santos NC, Figueira-Coelho J, Martins-Silva J, Saldanha C. “Multidisciplinary utilization of dimethyl sulfoxide: pharmacological, cellular, and molecular aspects.” Biochem Pharmacol. 2003 Apr 1; 65(7): 1035-41. Review. PMID: 12663039 [PubMed – indexed for MEDLIN
- Sethi N, Srivastava RK, Nath D, Singh RK. “Preclinical toxicity study of a male injectable antifertility agent (styrene maleic anhydride) in rhesus monkeys, Macaca mulatta.” J Med Primatol. 1991; 20(2): 89-93. PMID: 1865485 [PubMed – indexed for MEDLIN
- Sethi N, Srivastava RK, Nath D, Singh RK. “Teratological evaluation of an injectable male antifertility agent, styrene maleic anhydride, in rats.” Int J Fertil. 1992 May-Jun; 37(3): 183-7. PMID: 1355766 [PubMed – indexed for MEDLIN
- Sethi N, Srivastava RK, Singh RK, Bhatia GS, Sinha N. “Chronic toxicity of styrene maleic anhydride, a male contraceptive, in rhesus monkeys (Macaca mulatta).” Contraception. 1990 Sep; 42(3): 337-47. PMID: 2289393 [PubMed – indexed for MEDLIN
- Sethi N, Srivastava RK, Singh RK, Nath D. “Long-term toxicity studies of styrene maleic anhydride in rats.” Biomed Environ Sci. 1990 Dec; 3(4): 452-7. PMID: 2096850 [PubMed – indexed for MEDLIN
- Sethi N, Srivastava RK, Singh RK. “Histological changes in the vas deferens of rats after injection of a new male antifertility agent “SMA” and its reversibility.” Contraception. 1990 Mar; 41(3): 333-9. PMID: 2323221 [PubMed – indexed for MEDLIN
- Sethi N, Srivastava RK, Singh RK. “Male mediated teratogenic potential evaluation of new antifertility compound SMA in rabbit (Oryctolagus cuniculus).” Contraception. 1990 Aug; 42(2): 215-23. PMID: 2085972 [PubMed – indexed for MEDLIN
- Sethi N, Srivastava RK, Singh RK. “Safety evaluation of a male injectable antifertility agent, styrene maleic anhydride, in rats.” Contraception. 1989 Feb; 39(2): 217-26. PMID: 2706990 [PubMed – indexed for MEDLIN
- Sharma RS, Rajalakshmi M, Sharma RS, Jeyaraj DA. “Current status of fertility control methods in India.” J Biosci. 2001 Nov; 26(4 Suppl): 391-405. Review. PMID: 11779954 [PubMed – indexed for MEDLIN
- Sharma S, Sen P, Mukhopadhyay SN, Guha SK. “Microbicidal male contraceptive-Risug induced morphostructural damage in E. coli.” Colloids Surf B Biointerfaces. 2003 Oct; 32(1): 43-50.
Sharma U, Chaudhury K, Jagannathan NR, Guha SK. “A proton NMR study of the effect of a new intravasal injectable male contraceptive RISUG on seminal plasma metabolites.” Reproduction. 2001 Sep; 122(3): 431-6. PMID: 11597307 [PubMed – in process]
Sharma U, Chaudhury K, Jagannathan NR, Guha SK. “A proton NMR study of the effect of a new intravasal injectable male contraceptive RISUG on seminal plasma metabolites.” Reproduction. 2001 Sep; 122(3): 431-6. PMID: 11597307 [PubMed – indexed for MEDLIN - Singh AK. “Intra-vas deferens injection of styrene maleic anhydride gel for male contraception: is it safe?” J Fam Plann Reprod Health Care. 2002 Oct; 28(4): 208-9. No abstract available. PMID: 12419065 [PubMed – indexed for MEDLIN
- Singh H, Vasudevan P, Misro M, Ray AR, Guha SK. “Novel mode of contraception using polymeric hydrogels. I.” J Biomed Mater Res. 1982 Jan; 16(1): 3-9. PMID: 7056760 [PubMed – indexed for MEDLIN
- Toxicology Study Report – Shriram Institute for Industrial Research – Acute Toxicity LD50 Rat (intraperitoneal) Unpublished; available from Prof. Sujoy K. Guha.
Verma K, Misro MM, Singh H, Mahajan S, Ray AR, Guha SK. “Histology of the rat vas deferens after injection of a non-occlusive chemical contraceptive.” J Reprod Fertil. 1981 Nov; 63(2): 539-42. PMID: 7299756 [PubMed – indexed for MEDLIN
JQ1
JQ1 is a drug that targets the gene BRDT, which is responsible for sperm production. With the BRDT gene turned off, sperm production in the testes drops off with the remaining sperm losing motility.
In animal trials, the drug took about six weeks to demonstrate infertility and 1-3 months to regain fertility. The drug did not affect testosterone or mating patterns, but it did reduce teste size.
 JQ1’s initial findings occured during a cancer study. The drug still needs to be isolated into a lead compound for specific use as a contraceptive. It would then need to undergo toxicology before beginning clinical trials.
JQ1’s initial findings occured during a cancer study. The drug still needs to be isolated into a lead compound for specific use as a contraceptive. It would then need to undergo toxicology before beginning clinical trials.
Research Articles
- Bremner, W. J. (2012). Contraception for Men: A Breakthrough New Approach. Cell, 150(4), 667-668. [Preview Text]
- Fenner, A. (2012). Basic Research: Mouse Study Shows Spermatogenesis Inhibitor Is Effective as a Reversible Male Contraceptive. Nature Reviews Urology. 9, 544.
- Harrison, C. (2012). Epigenetic Targets: Towards a Male Contracceptive? Nature Reviews Drug Discovery. 11, 750.
























